
Did the COVID-19 crisis lead to an increased focus on competitiveness in healthcare? Maybe, although it was certainly no easy path. The pandemic represented tough choices for governments, societies, and companies. Especially healthcare and life science companies were forced to react as quickly as possible to minimize the impact of the crisis. The COVID-19 pandemic transformed the world. Better said, it accelerated changes already in development, leading to a process of industrial mutation. Health science companies around the world responded with an improved process structure, emerging stronger from the crisis than before.
Pharmaceutical, healthcare, and life sciences companies have played a crucial role in fighting against the COVID-19 pandemic. At the same time, the crisis demonstrated the ability of the sector to respond to an unexpected situation with unprecedented speed. For the first time in history, traditional healthcare and life science industry competitors collaborated to accelerate the research and development of the vital vaccine. At the same time, the crisis acted as a catalyst for the digital transformation in the healthcare industry. This positive ripple effect has led to digital disruption of the whole pharma sector, including biopharma and med-tech companies. As a result of this transformation, the role and impact of digitalization within the healthcare industry have shifted entirely. They may even have offered new possibilities in the life science sector altogether.
1: Digital Health Innovations
The past two years have been very challenging for the pharmaceutical industry. At the same time, the corona crisis demonstrated how important digitalization is for healthcare companies to remain viable and competitive in the future. One of the reasons for the rapid development of digital technologies was the effectiveness of modern technology, such as AI, predictive analytics, and other forms of digital technology in the fight against COVID-19. Even if there is nothing positive to be gained from the global pandemic, it has inevitably led to an overdue growth of digital technology in the healthcare industry. However, the sector still has a lot of untapped potentials to enhance, i.e., to increase the efficiency and flexibility of pharmaceutical production processes to respond more quickly to the market’s demands.
2: Research & development transformation
The average time to develop a drug is about 8.2 years (Source: Deloitte). For vaccines, it is even longer. Under normal circumstances, the research and development of a vaccine can take up to 10–15 years due to the high complexity of vaccine production. Driven by a global urgency, the pharma industry could get the COVID-19 vaccines developed, tested, and authorized in less than a year. As a result, companies focus on rethinking their processes to enhance efficiencies (Source: The History of Vaccines).
3: ESG Improvements
The crisis has also shifted the corporate focus on ESG (Environmental, Social, and Governance) factors, as the pandemic demonstrated the impacts of all types of risks on an industry. Companies that recognize the importance of sustainable practices will be more likely to drive sustainable growth and long-term competitive success. On the other hand, companies that neglect the importance of adapting to changing socio-economic and environmental conditions suffer financial consequences. An ESG strategy also includes increased transparency between the industry and the final consumer. (Source: Nasdaq).
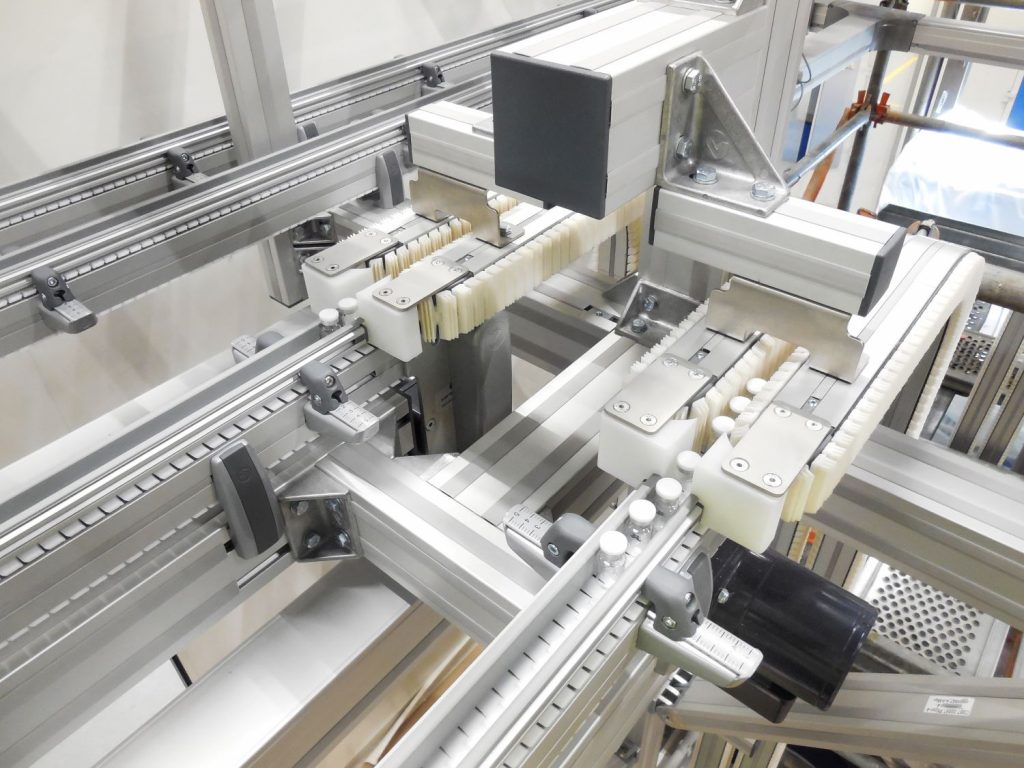
4: Solutions focused on efficiency
There were many lessons from COVID-19 for pharma companies. Still, the key to learning from the crisis was the focus on improving processes to resolve supply chain challenges and scale up production to ensure the global health products supply. Thus, automated production flow solutions are key to increasing the efficiency of pharmaceutical production processes. To ensure new products quickly reach people, manufacturers benefit from investing in a more extensive network of partners. For example, suppliers capable of implementing proven solutions worldwide with expertise in GMP/QSR regulated industries to help quickly ramp up local manufacturing capacity.
At FlexLink, we collaborate with leading machine and equipment providers in the healthcare industry to deliver proposals based on customer requirements. Last year, we helped a multinational in the pharmaceutical sector in Belgium to increase its yearly production capacity from 1.3 to 2 bn vaccine vials. The project was awarded to us after another supplier didn’t have the necessary experience to achieve the planned factory upgrade to allow delivering significantly more doses. The fast and professional implementation of this project was very important to our client to ensure there was no disruption of supply.
One of our strengths lies in the simulation of production lines, which allows testing the potential optimization to identify the right solution for the pharmaceutical production processes. The simulation also helps with a fast and trouble-free installation. Moreover, our new layouts fit more production capacity into a smaller footprint utilizing valuable floor space. The standardization makes machine integration and machine interface quick and easy. Another big benefit is related to the smart drive unit, which is an energy-efficient drive unit with embedded, easy-to-configure control features that do not require any programming. It is packed in a compact housing with connectors for sensors and actuators and industrial network connections. Each smart drive unit has enough processing capacity and intelligence to control local sensors, drive speed, and acceleration parameters.
Feel free to get in touch if you have questions about improving your pharmaceutical production efficiency. Also, read our other blogs related to the pharmaceutical industry here.




Leave a Reply