
At ACHEMA 2024, pharmaceutical Equipment manufacturers showcased their commitment to enhancing efficiency and ensuring compliance through advanced manufacturing processes and innovative solutions. The industry faces increasing pressure to meet stringent regulatory standards while optimizing production, reducing time to market, and controlling costs. This blog explores the challenges of regulatory pressures in the pharma industry, the critical importance of production efficiency, and how advanced technologies can address these demands effectively.
Navigating regulatory changes and market dynamics in the pharmaceutical industry
The pharmaceutical industry thrives on rapid innovation and intense competition. Trends such as biologics, personalized medicine, biosimilars, and digital health technologies necessitate continual investment in new technologies and adaptable processes to stay competitive.
In recent years, agencies like the FDA and EMA have driven significant changes in global pharmaceutical regulations. These changes emphasize data integrity, transparency, and patient-centered outcomes, mandating robust systems and adherence to Good Manufacturing Practice (GMP) guidelines. As a result, manufacturers must navigate heightened scrutiny, accelerate time to market, and manage costs without compromising quality or compliance.
Technological advancements
Automation, AI, and machine learning are pivotal in modern pharmaceutical manufacturing, enhancing efficiency, quality, and cost-effectiveness. Continuous manufacturing methods offer scalability and consistency. Advanced analytics provide real-time monitoring, enhancing decision-making and ensuring regulatory compliance.
Supply chain challenges and process optimization
Globalization has complicated the pharmaceutical supply chain, introducing security, sourcing, and geopolitical challenges. Strategies like diversifying suppliers, localizing manufacturing, and leveraging digital solutions are essential for enhancing supply chain resilience and visibility. Efficiency is critical in meeting regulatory requirements and market demands. Advanced automation and innovative solutions streamline operations, reduce downtime, and minimize errors. In pharmaceutical manufacturing, it’s crucial to handle sensitive products gently and maintain sterile conditions to meet healthcare sector expectations.
Buffering solutions
At ACHEMA 2024, FlexLink introduced an advanced buffering solution to tackle specific pharmaceutical manufacturing challenges. Pharmaceutical production demands high efficiency, precise handling, and contamination-free processing of delicate products like vials and ampoules. FlexLink’s buffering systems address these challenges by streamlining production processes. They efficiently sort, buffer, and distribute products, reducing downtime and minimizing errors. Buffering systems optimize production flow, reduce bottlenecks, and ensure a smooth manufacturing process. Engineered to optimize production flow and reduce bottlenecks, FlexLink’s buffering systems ensure a smooth manufacturing process. Key features include:
- Seamless integration: Our buffering systems are designed to integrate seamlessly with existing production lines, enhancing overall efficiency and reducing downtime.
- Precision and reliability: These systems offer precise handling and reliable performance, essential for maintaining product integrity and compliance with regulatory standards.
- Customizable solutions: FlexLink’s buffering systems are tailored to meet each manufacturer’s specific needs and provide customizable solutions that address unique production challenges.
Robotic Sorter Ring: maximize efficiency with FlexLink’s innovative sorting system
Tailored for pharmaceutical applications, this system integrates a patented ICS Rotating Table and the X45X Stainless Steel Conveyor. Additionally, it includes an RC12 Robotic Cell equipped with an Omron TM12 Collaborative Robot, ensuring unparalleled efficiency and flexibility. The ICS Rotating Table, a precision sorting and buffering system, is designed to streamline product distribution with utmost accuracy.
Integrated with the specialized X45X Stainless Steel Conveyor and the RC12 Robotic Cell, this system revolutionizes efficiency and flexibility in pharmaceutical production. The ICS rotating Table is the infeed system that efficiently sorts, buffers, and distributes products. The system seamlessly transfers products onto the X45X Stainless Steel Conveyor. Designed specifically for pharmaceutical applications, this conveyor ensures optimal handling of small products, enhanced cleanability, and reduced maintenance.
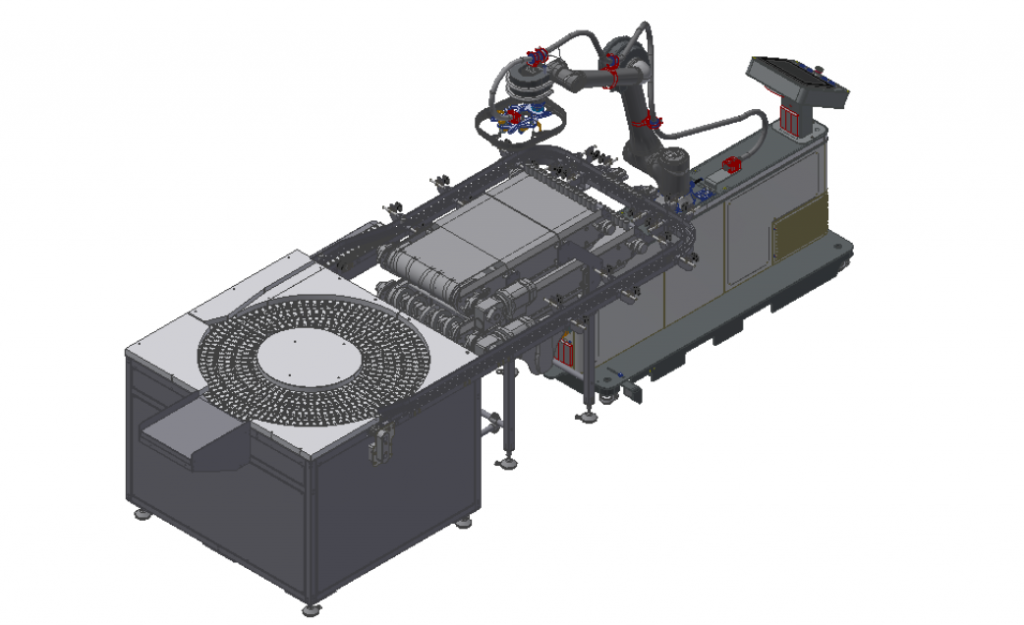
- Low noise levels: The Rotating Table operates with significantly lower noise levels than traditional rotating tables, creating a more comfortable and safer working environment.
- High flexibility: Designed with versatility in mind, the Rotating Table offers flexibility in size and overall dimensions, accommodating a wide range of production requirements.
- Gentle product handling: With minimal to no glass-to-glass contact, the Rotating Table ensures gentle handling of delicate products, reducing the risk of damage and contamination.
- Handling capability: The Rotating Table can manage products down to 16mm in diameter, making it ideal for various pharmaceutical applications, including small vials and ampoules.
FlexLink’s commitment to excellence and innovation empowers pharmaceutical manufacturers to navigate regulatory complexities confidently while ensuring product integrity and compliance. By adopting advanced technologies like buffering systems, manufacturers can achieve the efficiency needed to succeed in today’s regulatory landscape.
To explore how FlexLink’s solutions can enhance your pharmaceutical manufacturing processes, visit our website, attend industry events like ACHEMA, or contact our sales representatives for personalized consultations. Stay ahead with FlexLink’s innovative technologies and elevate your operations to meet the highest standards of efficiency and compliance. Also, don’t miss our other blogs about pharmaceutical manufacturing.



Leave a Reply